細胞株の詳細データ
TKG 0479 :: MCF7
| ID | TKG 0479 | ||||||||||||||||||
| Cell name | MCF7 | ||||||||||||||||||
| Data accepted | |||||||||||||||||||
| Animal | Human. | ||||||||||||||||||
| Scientific name | Homo sapiens. | ||||||||||||||||||
| Sex | |||||||||||||||||||
| Case history | |||||||||||||||||||
| Tissue | Breast adenocarcinoma. | ||||||||||||||||||
| Genetics | |||||||||||||||||||
| Life span | Infinite. | ||||||||||||||||||
| Morphology | Epithelial-like | ||||||||||||||||||
| Medium | E-MEM supplemented with non-essential AA, sodium pyruvate and 10% FBS, or RPMI-1640 + 10% FBS. | ||||||||||||||||||
| Passage method | 0.02% EDTA-PBS. | ||||||||||||||||||
| Characteristics | Established from pleural effusion of breast adenocarcinoma. Patint data: 69-y.o., female, Caucasian, O-type blood. This cell line retains several characteristics of differentiated mammary epithelium including ability to process estradiol via cytoplasmic estrogen receptors and forming domes. A culture submitted to the Cell Resource Center for Biomedical Research was found to be contaminated with mycoplasma. Progeny were cured by Treatments with BM Cyclin and MC210. | ||||||||||||||||||
| HLA profile | A2, A34, B12, B15 (HLA-A2/-, B18/44 and Cw0501/-). | ||||||||||||||||||
| CO2 concentration | 5% | ||||||||||||||||||
| Subculture frequency | 1:4, twice a week. | ||||||||||||||||||
| Classification | |||||||||||||||||||
| Mycoplasma | PCR: -, Hoechst DNA staining: - | ||||||||||||||||||
| Established by | H.D. Soule | ||||||||||||||||||
| References | J. Natl. Cancer Inst(Bethesda), 51, 1409-1416, 1973(PMID: 4357757)., Int. J. Cancer, 68, 535-546, 1996(PMID: 8945627). | ||||||||||||||||||
| References by users | Int. J. Cancer, 80, 64-67, 1999(PMID: 9935232)., Biochem. Biopys. Res. Commun., , 270, 649-656, 2000(PMID: 10753678)., J. Biol. Chem, 276, 42908-42914, 2001(PMID: 11555647)., Blood, 99, 4070-4078, 2002(PMID: 12010809)., Endocrinology, 143, 2635-2642, 2002(PMID: 12072396)., Toxicol. Lett., 143, 1-7, 2003(PMID: 12697374)., Biochem. Biophys. Res. Commun., 314, 805-809, 2004(PMID: 14741707)., Cancer Lett., 220, 197-210, 2005(PMID: 15766595)., Cancer Res., 66, 535-542, 2006(PMID: 16397270)., Cancer Res., 67, 3945-3954, 2007(PMID: 17440110)., Cell Signal., 19, 185-193, 2007(PMID: 16887331)., Cancer Res., 69, 1392-1399, 2009(PMID: 19190350)., Cancer Res., 70, 6659-6669, 2010(PMID: 20710045)., Oncol Rep. 26: 949-955, 2011 (PMID: 21743971), Cancer Med. 2013 Jun;2(3):305-15(PMID: 23930207), J Nat Prod. 2017 Feb 24;80(2):499-502(PMID: 28181805), Chem Pharm Bull. 2018;66(6):682-687(PMID: 29863070), J Radiat Res. 2020 Jul 6;61(4):524-534(PMID: 32367141), Biochem Biophys Rep. 2021 Jan 12:25:100902(PMID: 33490649), Oncol Rep. 2021 Jul;46(1):132(PMID: 34013368), Cardiovasc Toxicol. 2022 May;22(5):462-476(PMID: 35190965), Toxicol In Vitro. 2023 Dec:93:105698(PMID: 37739323). | ||||||||||||||||||
| Comments | |||||||||||||||||||
| Deposited by | |||||||||||||||||||
| Restriction | |||||||||||||||||||
| DNA Profile (STR-PCR) |
|
||||||||||||||||||
| Photo | 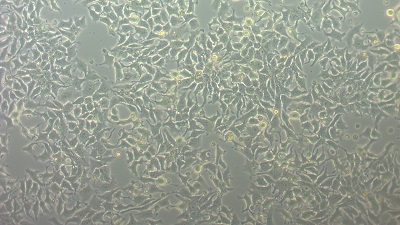 |

