Mechanisms of mitotic regulation in relation to oncogenesis and anti-cancer therapy
Cancer is characterized by unrestricted proliferation of cells. Our body is made up with sixty trillion cells as a result of proliferation (division) of one fertilized egg. In each cell of our body, 46 chromosomes are properly transmitted. On the other hand, most of the cancer cells show abnormalities in chromosome number and structure due to chromosomal instability, which is an increased rate of chromosome missegregation in mitosis. Chromosomal instability is not just a result of oncogenic transformation, but supposed to be closely related to oncogenesis and cancer progression, although its detailed mechanism is elusive. We are investigating how chromosomal instability occurs by elucidating the mechanisms of mitotic regulation. On the other hand, the mitotic phase is the time when many anti-cancer drugs show their effects on tumour cells, and we are aiming to develop a new strategy for anti-cancer therapy.
1) Molecular mechanism of chromosome segregation
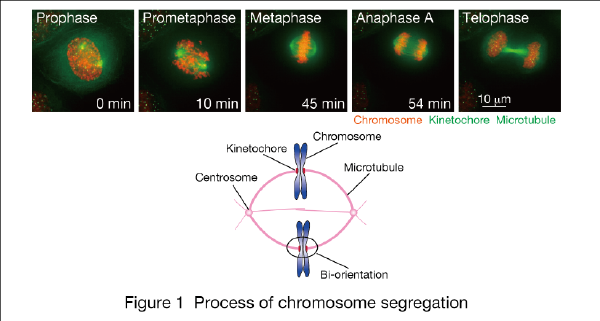
In mitotic phase, replicated chromosome pairs (sister chromatids) attach to microtubules from opposite spindle poles (bi-orientation), and segregate to the two daughter cells by being pulled towards respective spindle poles (Fig. 1). Control mechanisms like spindle checkpoint ensure proper chromosome segregation, and we are investigating these mechanisms.
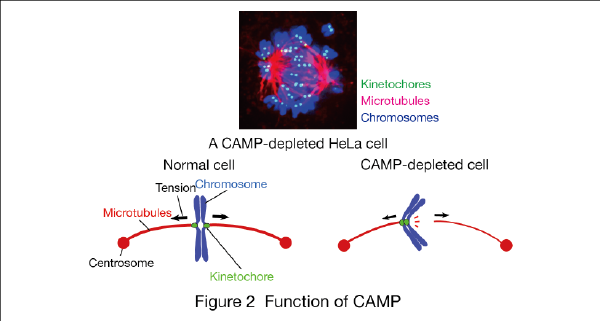
For example, we found a novel molecule involved in chromosome segregation, termed CAMP (Chromosome alignment-maintaining phosphoprotein). In CAMP-depleted cells, chromosomes are not segregated, and instead scattered along the spindle (Fig. 2). We found that CAMP is required for maintaining kientochore-microtubule attachment when tension is exerted between sister kinetochores (EMBO J 2011). We are now studying the effect of CAMP gene-knockout in mice.
We also found that Nup188, a nuclear pore complex component, localizes to centrosomes and works for chromosome segregation (Cancer Res 2013), and CLIP-170, a microtubule binding protein, recruits a mitotic kinase, Plk1, to kinetochores (J Cell Sci 2014).
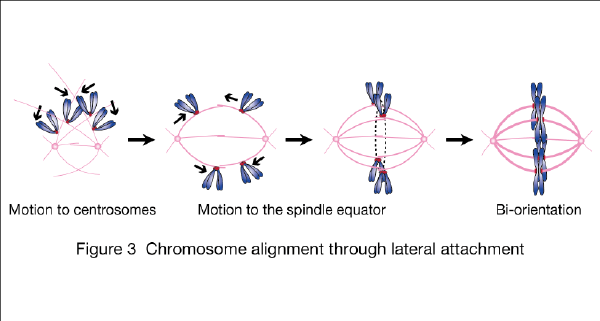
In addition, we are aiming to elucidate how chromosomes are captured by microtubules and establish bi-orientation on the spindle, based on the works in University of Dundee, Scotland, using budding yeast as a model organism (Nature 2005, J Cell Biol 2007, Dev Cell 2011). We are especially focusing on the process that kinetochores attach to microtubule lattice (lateral attachment; Figure 3) before attaching to microtubule ends (end-on attachment).
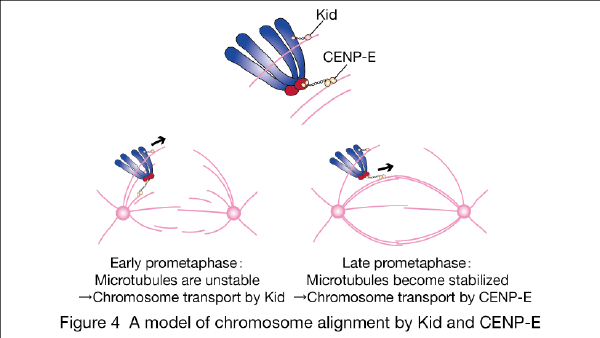
We recently found that two motor proteins, Kid and CENP-E, are working on the alignment of laterally-attached chromosomes to the spindle equator (Figure 4; Nat Commun 2015).
We are also studying whether the defects in these mechanisms for proper chromosome segregation lead to chromosomal instability, elucidating the relevance to oncogenesis.
2) Development of anti-mitotic drugs
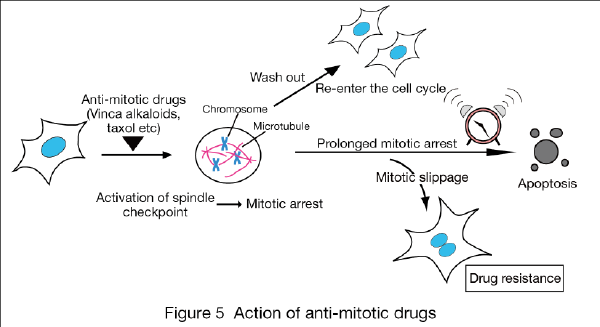
Anti-cancer drugs like the vinca alkaloids and taxol are widely used for the treatment of various cancers such as malignant lymphoma and breast cancer. These drugs prevent proper attachment of chromosomes to microtubules and thus activate spindle checkpoint (Fig. 5). Sustained spindle checkpoint activation is thought to induce cell death, although its mechanism is largely unknown. We reported that a similar mechanism exists to induce cell death in budding yeast (FEBS Lett 2010). In contrast, a fraction of cells escape from mitotic arrest and re-enter the cell cycle (mitotic slippage), which leads to drug resistance. Using human cells, now we are developing novel anti-cancer drugs that efficiently induce cell death after mitotic arrest, and thus can avoid drug resistance.
Chromosome segregation is a very dynamic process, and has been attracted scientists for over hundred years. We try to build up and verify a hypothesis through our observation of chromosome motion, avoiding preconceptions. Through the studies mentioned above, we are aiming to contribute to the elucidation of mechanisms of oncogenesis and resistance to anti-cancer drugs, prediction of drug effects on individual patients, and modification of drug effects.

 Home
Home